Welcome to the Romanian Nagoya Protocol Tools
This platform is dedicated to the implementation of the Nagoya Protocol and ABS Regulation in Romania
BASIC INFORMATION
The national platform was created within the project "Consolidation of the institutional capacities of the Ministry of the Environment and subordinate units for the creation of policies in the field of biodiversity - financing contract 455/18.12.2019, code SIPOCA 594/code MYSMIS 127465
Article 1 of the Convention on Biological Diversity (CBD) states that "fair and equitable sharing of the benefits arising out of the utilisation of genetic resources" is a fundamental component of strategies for the conservation and sustainable use of biodiversity. At the end of the 10 th Meeting of the Conference of the Parties to the CBD held on 29 October 2010 in Nagoya, Japan, the Nagoya Protocol on Access to Genetic Resources and Fair and Equitable Sharing of the Benefits Arising out of their Utilization, a supplementary agreement to the Convention on Biological Diversity (hereafter referred to as the "Nagoya Protocol", according to international nomenclature) was adopted.
The Nagoya Protocol is a legal instrument developed out of the need to implement and clarify one of the articles of the CBD, namely Article 15 on "access to genetic resources" and "fair and equitable sharing of the benefits" arising from the use of these resources. The Protocol establishes the international legal framework to promote the transparent and effective implementation of the concept of access to genetic resources and the sharing of benefits arising from the utilisation of these resources at national, regional and local levels.
At the national level, the legislative aspects of the ratification and national transposition of the Nagoya Protocol were materialized in Law No 36 of 17 January 2019 for the ratification of the Nagoya Protocol on Access to Genetic Resources and Fair and Equitable Sharing of Benefits Arising out of their Utilization, adopted in Nagoya on 29 October 2010, signed by Romania on 20 September 2011 in New York, as a supplementary agreement to the Convention on Biological Diversity, originally signed on 5 June 1992 in Rio de Janeiro.
In Romania, the implementation of the Nagoya Protocol was developed starting from the analysis of its implementation at EU level and the identification of specificities in the application of the Protocol's provisions in a participatory process at national level.
Romania, as a signatory to the Nagoya Protocol and EU member state, according to the EU ABS Regulation No 511/2014, establishes through its own national regulations the ownership of genetic resources, as well as the contact points and competent national authorities, i.e. the authorities that have the obligation to inform and grant access to potential users to genetic resources and/or associated traditional knowledge on the national territory.
In Romania, access to genetic resources of wild fauna and flora, as well as to genetic resources in collections, is subject to obtaining prior informed consent (PIC), mutually agreed terms (MAT) and Access Permit (Internationally Recognized Certificate of Compliance - IRCC), as appropriate.
The Nagoya National Platform is administered by the Competent National Authority (CNA) – the National Environmental Protection Agency. The National Focal Point (NFP) registers in the Access and Benefit-sharing Clearing-House (ABS-CH) platform the access permits issued by the CNA to become IRCCs. In the process of monitoring the use of genetic resources, the NFP communicates with third parties through the Checkpoint Communiqués (CPC) where the use of genetic resources accessed from Romania is taking place..
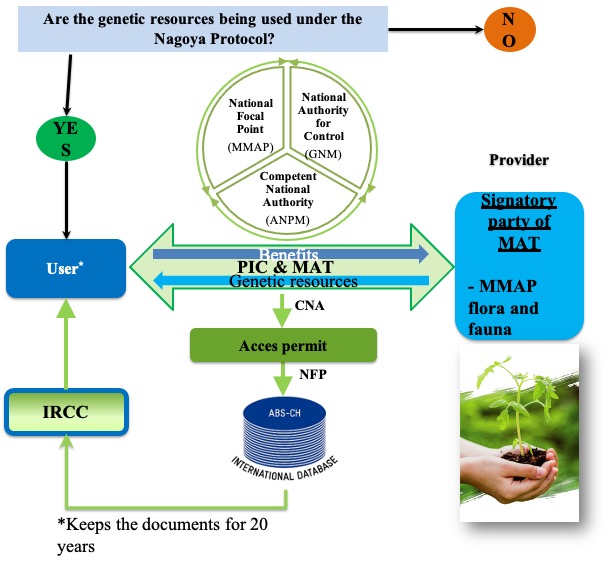
In Romania, all the requesting access to genetic resources regulated by other international agreements that are complementary to the objectives of the CBD and the Nagoya Protocol, as well as other specific situations, are exempted from applying the provisions of the Nagoya Protocol and EU Regulation 511/2014. In order to select applications that do not fall under the Protocol, the user, after registering on the platform, is asked to answer a series of questions as follows:
1. Are genetic resources acquired before the entry into force of the Nagoya Protocol?
The EU ABS Regulation applies from 12 October 2014, which is the date when the Nagoya Protocol entered into force for the Union. Genetic resources accessed prior to that date fall outside the scope of the Regulation even if utilisation of those resources occurs after 12 October 2014 (see Article 2(1) of the Regulation 511/2014). In other words, the Regulation only applies to genetic resources which were accessed as of 12 October 2014.
2. The requested access refers to human genetic resources?
Human genetic resources are out of scope of the EU Regulation 511/2014 because they are not covered by the CBD and the Protocol. This is confirmed by CBD COP Decision II/11 (para. 2) and CBD COP Decision X/1 (para. 5, specifically for ABS).
3. The genetic resources you want to access are marine genetic resources from areas beyond national jurisdiction?
Genetic resources from high seas (“parts of the sea that are not included in the exclusive economic zone, in the territorial sea or in the internal waters of a State, or in the archipelagic waters of an archipelagic State” (UNCLOS, Part VII, Section 1, Article 86)) or the Area (“the seabed and ocean floor and subsoil thereof, beyond the limits of national jurisdiction” (UNCLOS, Part I, Article 1, 1(1)), are exempted from applying the provisions of the Nagoya Protocol. This category of resources is regulated by United Nations Convention on the Law of the Sea (UNCLOS).
4. The requested access refers to genetic resources originating from the Antarctic region (60-90 degrees south latitude)?
Genetic resources originating from the Antarctic region (60-90 degrees south latitude) are outside of scope of Nagoya Protocol because are covered by Antarctic Treaty System.
5. The genetic resources you want to access are “PIP Biological Materials” in accordance with the purpose, term of reference and provisions of the “Who pandemic influenza preparedness framework” (PIPF)?
“PIP Biological Materials” refers to the genetic resources that are included in one of the following categories:
-virus isolates of wild type human H5N1 and other influenza viruses with human pandemic potential; and modified viruses prepared from H5N1 and/or other influenza viruses with human pandemic potential developed by WHO GISRS* laboratories;
-RNA extracted from wild-type H5N1 and other human influenza viruses with human pandemic potential and cDNA that encompass the entire coding region of one or more viral genes;
- “Clinical specimens” that means materials taken from humans or animals, in as far as the samples taken from animals are shared by originating countries/laboratories with the WHO GISRS*. These include specimens collected from the respiratory tract (for example, swabs and aspirated fluid), and also blood, serum, plasma, faeces, and tissues, for diagnostic purposes, detection of pathogens and further characterization, study or analysis.
“PIP Biological Materials” are regulated by Pandemic Influenza Preparedness Framework for the Sharing of Influenza Viruses and Access to Vaccines and Other Benefits (PIPF) and do not fall under Nagoya Protocol.
6.The requested access concerns genetic resources used as bulk commodities?
According to C 13/01, 12.01.2021, Commission notice — Guidance document on the scope of application and core obligations of Regulation (EU) No 511/2014 of the European Parliament and of the Council on the compliance measures for users from the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization in the Union, Paragraph 2.3.1.3.: “Trade and exchange of genetic resources as commodities (such as agricultural, fisheries or forestry products — whether for direct consumption or as ingredients, e.g. in food and drink products) fall outside the scope of the Regulation. The Protocol does not regulate issues related to trade, but is applicable only to utilisation of genetic resources. As long as there is no research and development on genetic resources (thus no utilisation in the sense of the Protocol), the EU ABS Regulation does not apply.
7. The access is required for genetic resources further used for trade?
According to C 13/01, 12.01.2021, Commission notice — Guidance document on the scope of application and core obligations of Regulation (EU) No 511/2014 of the European Parliament and of the Council on the compliance measures for users from the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization in the Union, Paragraph 2.3.1.3.: “The Protocol does not regulate issues related to trade, but is applicable only to utilization of genetic resources. As long as there is no research and development on genetic resources (thus no utilization in the sense of the Protocol), the EU ABS Regulation does not apply.”
8. The requested access refers to derivatives that will be accessed independently of the genetic resources and there is no ascertainable level of continuity between the derivative and the genetic resource from which it was obtained?
In C 13/01, 12.01.2021, Commission notice — Guidance document on the scope of application and core obligations of Regulation (EU) No 511/2014 of the European Parliament and of the Council on the compliance measures for users from the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization in the Union, Paragraph 2.3.4. – the access of derivatives is clearly explained: “The definition of utilization in the Protocol and the Regulation applies to ‘research and development on the genetic and/or biochemical composition of genetic resources, including through the application of biotechnology’. Biotechnology, in turn, is defined in the CBD as ‘any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for specific use’ (Article 2, see also Article 2(d) of the Protocol). Thus, through the concept of ‘biotechnology’, the definition of utilization is interlinked with the definition of ‘derivatives’ in Article 2(e) of the Protocol, which clarifies that ‘derivative’ means ‘a naturally occurring biochemical compound resulting from the genetic expression or metabolism of biological or genetic resources, even if it does not contain functional units of heredity’. Examples of derivatives include proteins, lipids, enzymes, RNA and organic compounds such as flavonoids, essential oils or resins from plants. Some such derivatives may no longer contain functional units of heredity. However, as the reference to naturally occurring biochemical compounds makes clear, the definition does not cover material such as synthetic gene segments. Derivatives are referred to in the definition of biotechnology, which in turn is mentioned in the definition of utilisation, but no corresponding reference is to be found in the substantive provisions of the Protocol, including those related to utilization, which ultimately determine its scope of application. Consequently, access to derivatives is covered by the EU ABS Regulation when it also includes genetic resources for utilization, e.g. when access to a derivative is combined with access to a genetic resource from which that derivative was or is obtained or when research and development to be carried out on such derivatives is addressed in mutually agreed terms transferred to the user.
In other words, there needs to be an ascertainable level of continuity between a derivative and the genetic resource from which it was obtained for research and development activities on derivatives to fall in the scope of the EU ABS Regulation.”
9. The genetic resources you want to access originate from a new variety of plants?
According to In C 13/01, 12.01.2021, Commission notice — Guidance document on the scope of application and core obligations of Regulation (EU) No 511/2014 of the European Parliament and of the Council on the compliance measures for users from the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization in the Union, Paragraph 5.2.2.: ”The UPOV Convention does not constitute a specialized ABS instrument in the meaning of Article 4(4) of the Protocol. However, the Nagoya Protocol makes it clear – and the EU ABS Regulation confirms this (see Recital 14) – that it should be implemented in a manner which is mutually supportive with other international agreements, provided they are supportive of and do not run counter the objectives of the Convention on Biological Diversity and the Nagoya Protocol. Furthermore, Article 4(1) of the Protocol provides that it does not affect the rights and obligations derived from existing international agreements (if they do not pose a serious damage or threat to biological diversity). The EU ABS Regulation is respectful of UPOV obligations: the compliance with the duties stemming from the Regulation does not conflict with the UPOV obligation to provide for the breeders exemption. In other words, the duty to apply due diligence is not in conflict with the ongoing use of material protected under the UPOV plant breeders' rights regime and coming from Parties to UPOV ”
The International Union for the Protection of New Varieties of Plants (UPOV) is an intergovernmental organization based in Geneva, Switzerland. UPOV was established in 1961 by the International Convention for the Protection of New Varieties of Plants (the "UPOV Convention") (www.upov.int).
The UPOV Convention was first signed in Paris in 1961, and revised in 1972, 1978 and 1991. The latest revision (the 1991 Act) entered into force in 1998 (https://www.upov.int/edocs/pubdocs/en/upov_pub_221.pdf).
The mission of UPOV is to provide and promote an effective system of plant variety protection, with the aim of encouraging the development of new varieties of plants, for the benefit of society.
Romania accedes to the convention by Law No. 186/2000 and becomes a member of UPOV in March 2001 (https://www.upov.int/edocs/pubdocs/en/upov_pub_423.pdf). The UPOV is implemented at national level by Law No. 204/2011.
The national legislation (https://upovlex.upov.int/en/legislation/profile/RO) provides that breeder’s rights in the new plant varieties of all genera and species of plants, including, among others, the hybrids between genera and species, are protected, recognized and defended on the territory of Romania, through the grant of a variety patent by the State Institute for Testing and Registration of Varieties (ISTIS) (https://istis.ro/en/).
According to Article 30 of the national legislation, the variety patent holder shall enjoy the exclusive right of exploitation of the new variety and the right to prevent any person, without his authorization, from performing the following acts in relation to the propagating material and harvested material of the protected variety.
In conclusion, genetic resources that originate from a new variety of plants are exempted from the provisions of the Nagoya Protocol
10. Are the plant genetic resources you want to access used for food and agriculture?
According to In C 13/01, 12.01.2021, Commission notice — Guidance document on the scope of application and core obligations of Regulation (EU) No 511/2014 of the European Parliament and of the Council on the compliance measures for users from the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization in the Union, Paragraph 2.3.1.1.: “In accordance with Article 4(4) of the Nagoya Protocol, specialized ABS instruments prevail in respect of the specific genetic resource covered by the specialized instrument and for the purpose of that instrument, if it is consistent with and does not run counter to the objectives of the CBD and the Protocol. Accordingly, Article 2(2) of the EU ABS Regulation makes it clear that the Regulation does not apply to genetic resources for which access and benefit-sharing is governed by such specialized international instruments. This currently includes material covered by the International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA) and the WHO’s Pandemic Influenza Preparedness (PIP) Framework.” As consequence, the use of genetic resources for food and agriculture are regulated by International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA) and do not fall into the scope of Nagoya Protocol.
11. The requested resources will be used for research and development on genetic and/or biochemical composition of genetic resources, including through the application of biotechnology?
‘Utilization of genetic resources’ is defined in the Regulation, exactly as in the Protocol, as „to conduct research and development on the genetic and/or biochemical composition of genetic resources, including through the application of biotechnology, as defined in Article 2 of the Convention” (Article 3(5) of the Regulation). If the utilization of genetic resources is not conducted as defined in the text of the Protocol, it does not fall into its scope.
12. The requested genetic resources will be used for basic/fundamental research activities without any application or direct commercial use?
In Romania, all the genetic resources required for basic research only, do not fall under the scope of the Nagoya Protocol.
According to The OECD's 2002 Frascati Manual „Basic research is experimental or theoretical work undertaken primarily to acquire new knowledge of the underlying foundation of phenomena and observable facts, without any particular application or use in view. Basic research analyses properties, structures and relationships with a view to formulating and testing hypotheses, theories or laws. The reference to no “particular application in view” in the definition of basic research is crucial, as the performer may not know about actual applications when doing the research or responding to survey questionnaires”
13.The basic research creates new insight into characteristics of the genetic resource which is of (potential) benefit to the further process of product development?
In Romania, all the genetic resources required for basic research only, do not fall under the scope of the Nagoya Protocol.
According to In C 13/01, 12.01.2021, Commission notice — Guidance document on the scope of application and core obligations of Regulation (EU) No 511/2014 of the European Parliament and of the Council on the compliance measures for users from the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization in the Union, Paragraph 2.3.3.1.: „The definition of utilization of genetic resources, i.e. to conduct research and development on the genetic and/or biochemical composition of genetic resources, is thus understood to apply to research and development on gene function and inheritable traits. As a type of ‘litmus test’, users should ask themselves whether what they are doing with the genetic resources creates new insight into characteristics of the genetic resource which is of (potential) benefit to the further process of product development. If this is the case, the activity goes beyond mere description, should be considered research and development and therefore falls under the term ‘utilisation’.” As consequence, if the utilization does not create new insight into characteristics of the genetic resource which is of (potential) benefit to the further process of product development then is exempted from the provisions of the Nagoya Protocol.
In addition, in Romania the utilisation of genetic resources originates from domestic animals, cultivated plants and microorganisms that are not included in collections are exempted from provisions of the Nagoya Protocol and EU Regulation 511/2014.